Preview 1
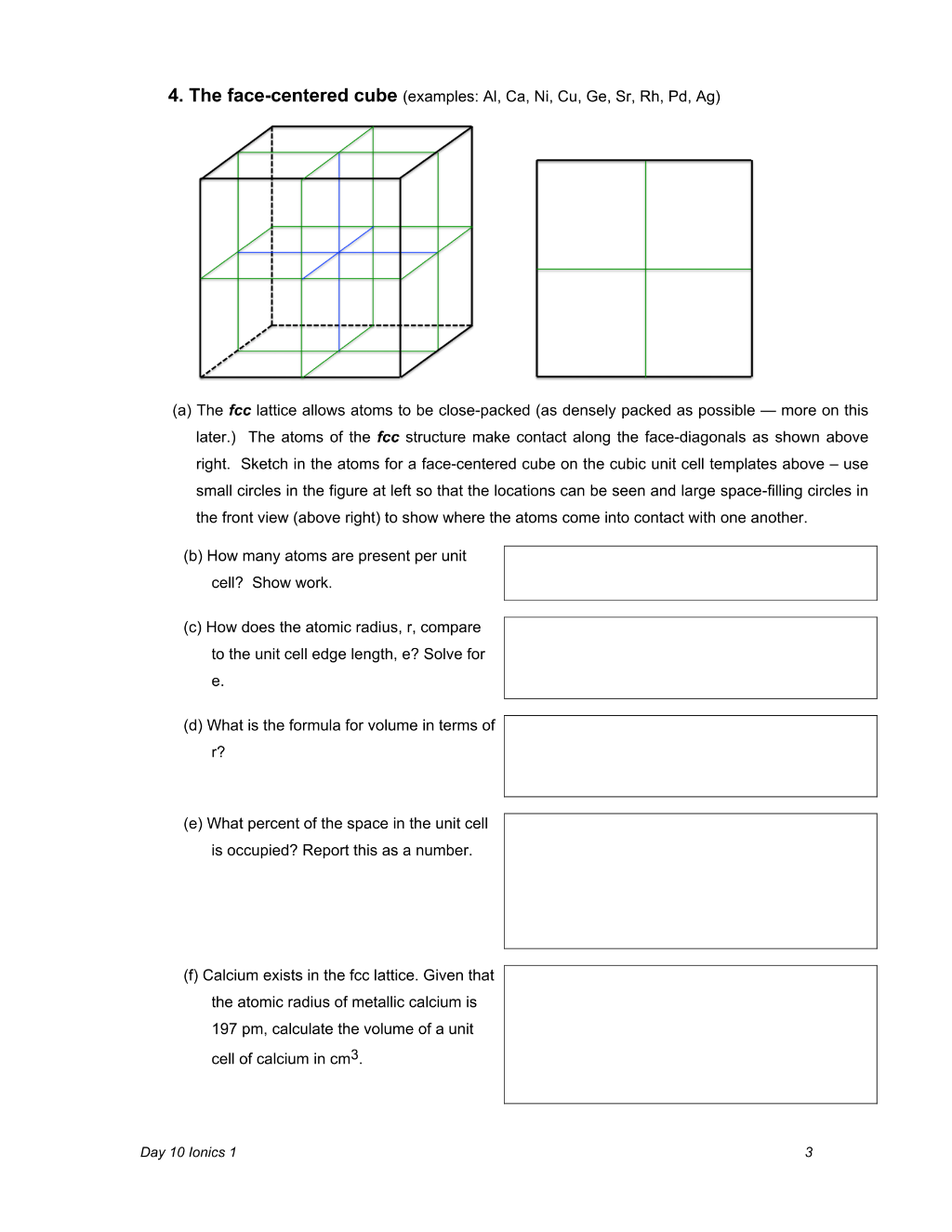
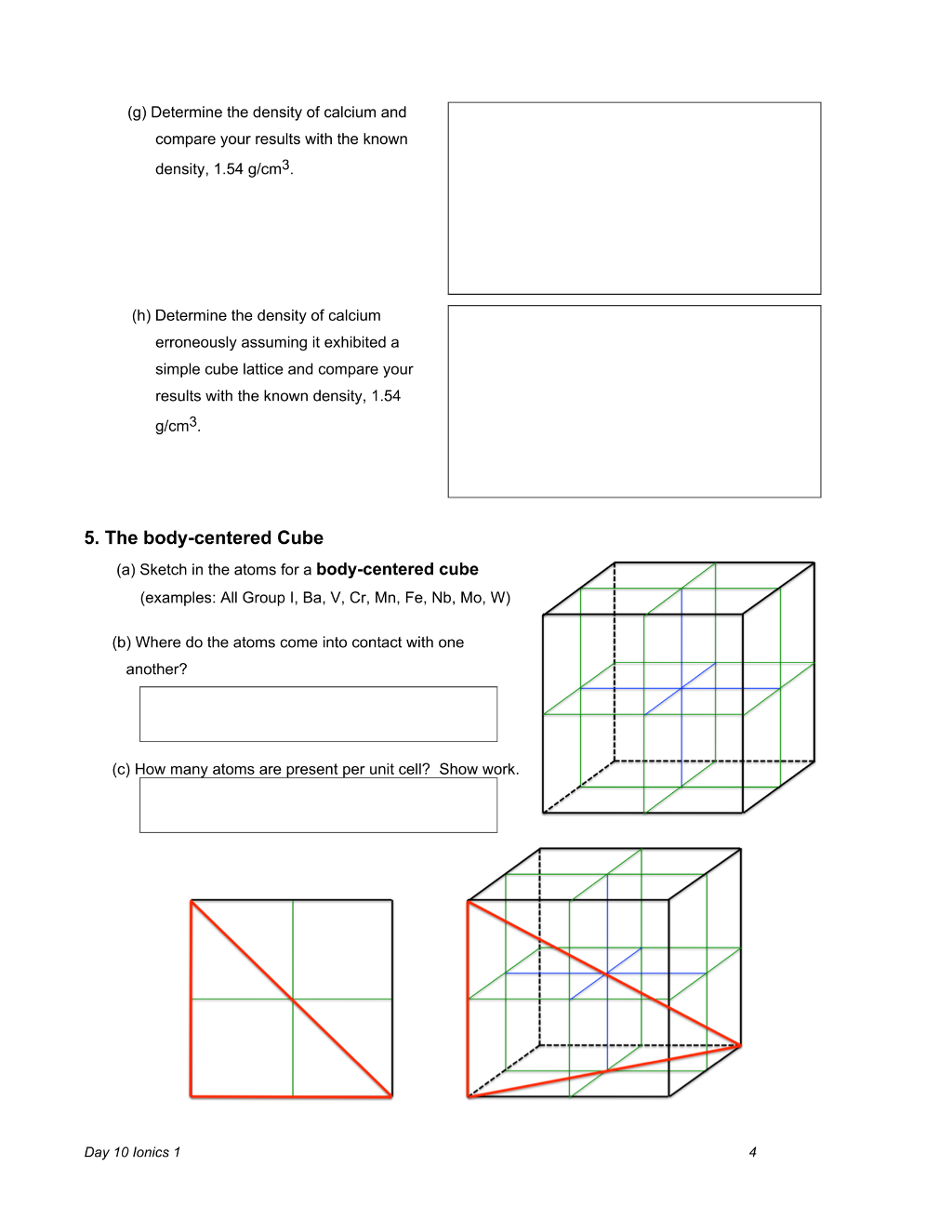
Day 10 Ionics 1 3 4. The face-centered cube (examples: Al, Ca, Ni, Cu, Ge, Sr, Rh, Pd, Ag) (a) The fcc lattice allows atoms to be close-packed (as densely packed as possible — more on this later.) The atoms of the fcc structure make contact along the face-diagonals as shown above right. Sketch in the atoms for a face-centered cube on the cubic unit cell templates above – use
Preview 1

Preview 2
Preview 3
Preview 4
Preview 5
Preview 6